Attachment Inhibitors and Post-attachment Inhibitors
Reviewed by: HU Medical Review Board | Last reviewed: May 2025 | Last updated: May 2025
Attachment inhibitors and post-attachment inhibitors are 2 classes of drugs used to suppress HIV in the body. When they are used in combination with other HIV-fighting drugs, the treatment regimen is referred to as antiretroviral therapy (ART).1
HIV needs to enter human host cells called CD4 cells, or T cells (which are a type of immune cell that HIV targets), to replicate. To enter into these cells, HIV needs to bind to the host cell surface and then fuse itself with the host cell to get inside.1
Attachment inhibitors bind to the gp120 protein on the outer surface of HIV, preventing HIV from entering CD4 cells.2
Post-attachment inhibitors block the binding step in the process, preventing HIV from attaching and getting into its target cell to replicate.1
HIV life cycle
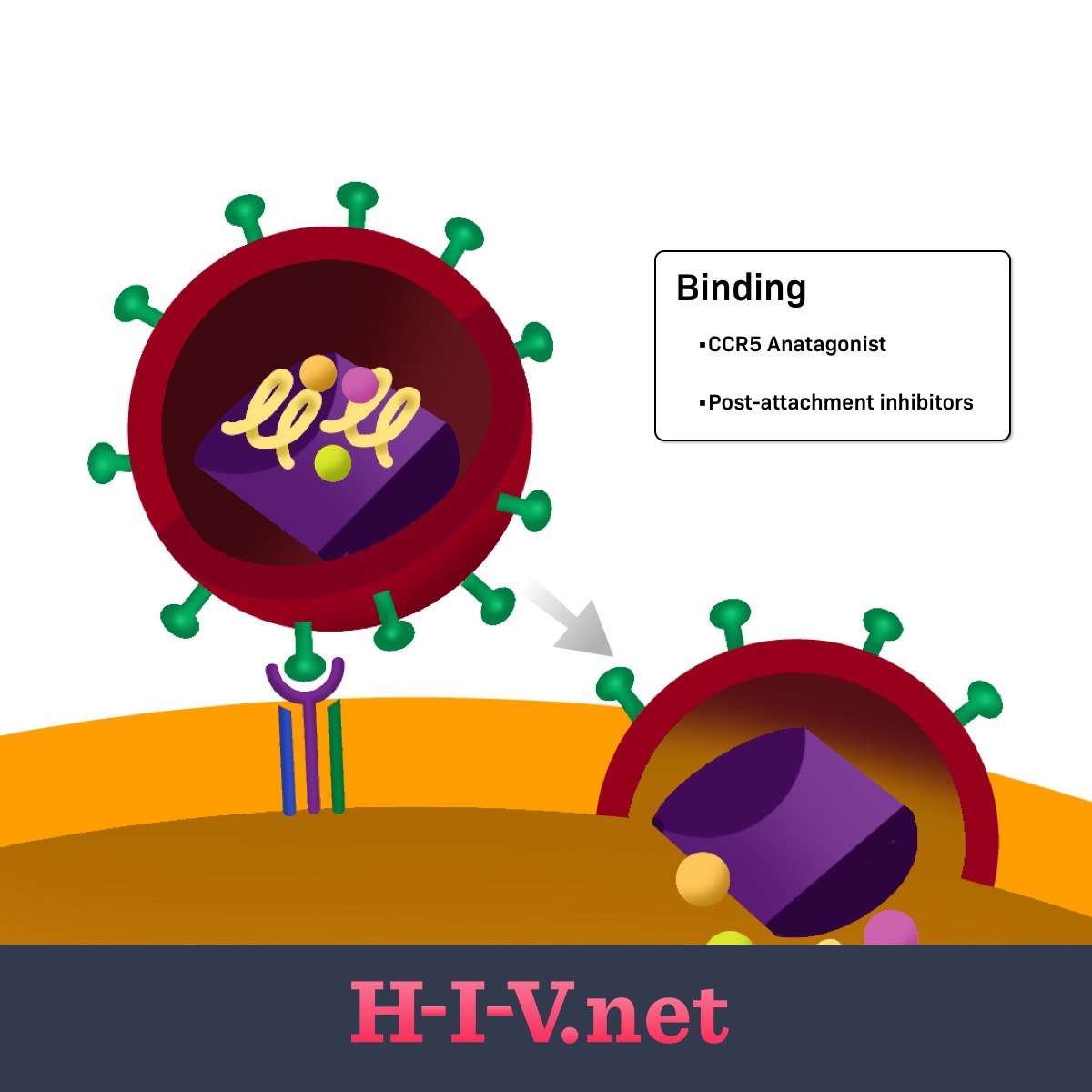
Viruses like HIV need human host cells to replicate. They cannot multiply on their own without human cells. When HIV particles called virions enter the body after a transmission event, the next main steps of the HIV lifecycle are as follows:3
- Binding
- Fusion
- Reverse transcription
- Integration
- Replication
- Assembly
- Budding
Figure 1. Post-attachment inhibitors target binding in the HIV life cycle
The HIV virions have proteins on the outside that recognize receptors on CD4 cells. Once a virion finds a CD4 cell, its outside proteins can bind to the CD4 cell receptor and link the CD4 cell and virion together (step 1: binding). For the virus to fully bind and fuse with the CD4 cell and get inside the cell to continue replication (step 2: fusion), other receptors on the CD4 cell surface may get involved.3
These additional receptors on the CD4 cell include the CCR5 or CXCR4 receptors. The HIV surface receptors involved in the first two steps are called gp120 and gp41. Any of these receptors on the CD4 cell or HIV itself may be targets for HIV drugs.3
Once inside the cell, the virion disassembles itself so that it can begin the replication process. The next three steps represent the replication process of the virus’s genetic material. The last two steps of the process involve HIV re-assembling itself into new, mature virions that can be released from the CD4 cell and enter the bloodstream where they can go on to infect new cells.3
How do attachment inhibitors work?
Attachment inhibitors work by blocking the virus from attaching to CD4 cells. To infect a cell, HIV needs to bind to specific receptors on the CD4 cell's surface. Attachment inhibitors prevent this binding process, essentially stopping the virus from entering the cell and replicating.2,4
By preventing the virus from entering healthy cells, these drugs help to lower the viral load in the body and slow the progression of HIV infection.2,4
Example of attachment inhibitors
Attachment inhibitors approved to treat HIV include:1,4
- Rukobia (fostemsavir)
How do post-attachment inhibitors work?
Post-attachment inhibitors block the CD4 receptor on the outside of CD4 cells. The CD4 receptor is involved in the binding of HIV to its target cell. HIV needs to bind using the CD4 receptor to move on to the next step in the life cycle, fusion, and entry into the cell. When the CD4 receptors are blocked by a post-attachment inhibitor, HIV cannot adequately bind to its target cell. This stops the replication process since HIV needs to get inside the human host cells to replicate.1,5
Think of it like a key trying to open a lock. Post-attachment inhibitors let the key (HIV) get partially into the keyhole (the CD4 cell receptor), but then the drug jams the lock after the key is inserted, preventing it from turning and opening the door (fusion and entry). They stop the virus from taking the necessary next step to fully enter the cell after its initial connection.1,5
Examples of post-attachment inhibitors
Post-attachment inhibitors approved to treat HIV include:1,5
- Trogarzo® (ibalizumab-uiyk)
What are the possible side effects?
The most common side effects of Trogarzo and Rukobia include:3
- Diarrhea
- Nausea
- Dizziness
- Rash
These are not all the possible side effects of attachment inhibitors and post-attachment inhibitors. Talk to your doctor about what to expect when taking these drugs. You also should call your doctor if you have any changes that concern you when taking attachment inhibitors and post-attachment inhibitors.
Other things to know
Before starting treatment with Trogarzo or Rukobia, tell your doctor if you are pregnant, planning to become pregnant, or breastfeeding.3,4
Before beginning treatment for HIV, tell your doctor about all your health conditions and any other drugs, vitamins, or supplements you take. This includes over-the-counter drugs.